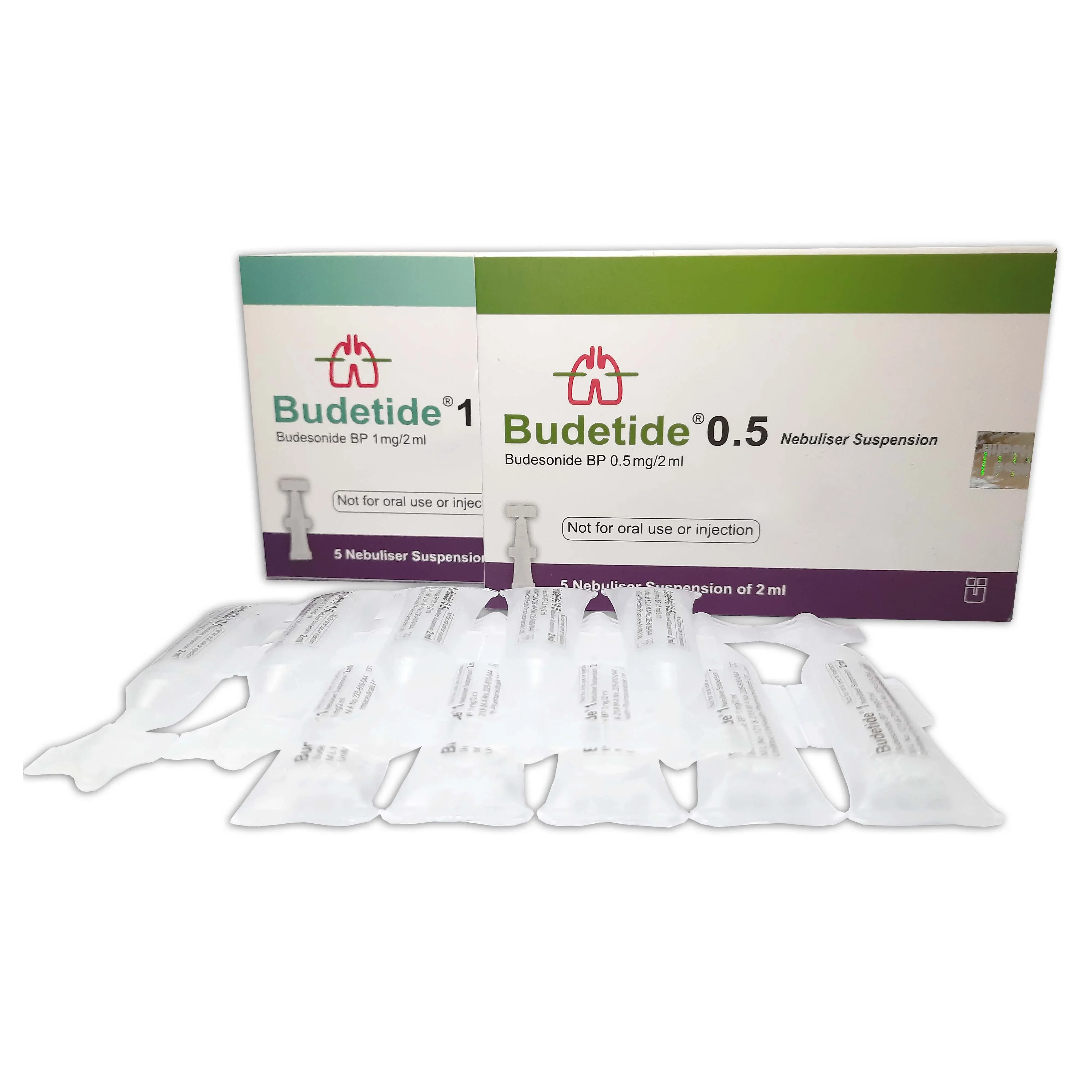
Budetide Nebuliser Suspension
Pack Image
0.5 mg/2 ml
2 ml ampoule:
৳ 40.00
(1 x 5: ৳ 200.00)
Also available as:
Indications
Treatment of persistent bronchial asthma in patients where use of a pressurised inhaler or dry powder formulation is unsatisfactory or inappropriate. Very serious pseudocroup (laryngitis subglottica) in which hospitalisation is indicated.
Pharmacology
Budesonide is a synthetic corticosteroid having potent glucocorticoid activity and weak mineralocorticoid activity. It has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol. Corticosteroids have been shown to have a wide range of inhibitory activities against multiple cell types (e.g., mast cell, eosinophil, neutrophil, macrophage, and lymphocyte) and mediators (e.g., histamine, eicosanoids, leukotriene, and cytokine) involved in allergic mediated inflammation.
Dosage
Asthma: The dose should be given twice daily. Administration once daily may be considered in cases of mild to moderate stable asthma.
Initial dosage: The initial dose should be tailored to the severity of the disease and thereafter should be adjusted on an individual basis. The following doses are recommended but the minimum effective dose should always be sought.
Onset of effect: An improvement of the asthma following administration of budesonide may occur within 3 days after initiation of therapy. The maximum effect will only be obtained after 2-4 weeks of treatment.
Patients in maintenance therapy with oral glucocorticosteroids:
Asthma: Budesonide Nebuliser suspension may permit replacement or significant reduction in dosage of oral glucocorticosteroids while maintaining asthma control. When transferral from oral steroids to budesonide Nebuliser suspension is started, the patient should be in a relatively stable phase. A high dose of budesonide Nebuliser suspension is then given in combination with the previously used oral steroid dose for about 10 days.
After that, the oral steroid dose should be gradually reduced (by for example 2.5 milligrams prednisolone or the equivalent each month) to the lowest possible level. In many cases, it is possible to completely substitute the oral steroid with budesonide Nebuliser suspension. For further information on the withdrawal of corticosteroids, when tapering off systemic corticosteroids some patients will experience steroid withdrawal symptoms, e.g. joint and/or muscle pain, lack of energy and depression or even a decreased lung function. Such patients must be advised to continue the inhaled budesonide therapy, but they should also be examined for any objective signs of adrenocortical insufficiency. If such signs are present, the dose of the systemic corticosteroid should be temporarily increased and then tapered off even more slowly. In periods of stress or severe asthma attacks, patients in the transition phase may require treatment with systemic corticosteroids.
Pseudocroup: In infants and children with pseudocroup, the commonly used dose is 2 mg of nebulised budesonide. This is given as a single administration, or as two 1 mg doses separated by 30 minutes. Dosing can be repeated every 12 hours for a maximum of 36 hours or until clinical improvement.
Initial dosage: The initial dose should be tailored to the severity of the disease and thereafter should be adjusted on an individual basis. The following doses are recommended but the minimum effective dose should always be sought.
- Children aged 6 months and above: 0.25-1.0 mg daily. For patients in maintenance therapy with oral steroids a higher initial dosage up to 2.0 mg daily should be considered.
- Adults (including the elderly) and children/adolescents over 12 years of age: 0.5-2 mg daily. In very severe cases the dosage may be increased further.
- Children aged 6 months and above: 0.25-1.0mg daily.
- Adults (including the elderly) and children/adolescents over 12 years of age: 0.5-2.0 mg daily. In very severe cases the dose may be further increased.
Onset of effect: An improvement of the asthma following administration of budesonide may occur within 3 days after initiation of therapy. The maximum effect will only be obtained after 2-4 weeks of treatment.
Patients in maintenance therapy with oral glucocorticosteroids:
Asthma: Budesonide Nebuliser suspension may permit replacement or significant reduction in dosage of oral glucocorticosteroids while maintaining asthma control. When transferral from oral steroids to budesonide Nebuliser suspension is started, the patient should be in a relatively stable phase. A high dose of budesonide Nebuliser suspension is then given in combination with the previously used oral steroid dose for about 10 days.
After that, the oral steroid dose should be gradually reduced (by for example 2.5 milligrams prednisolone or the equivalent each month) to the lowest possible level. In many cases, it is possible to completely substitute the oral steroid with budesonide Nebuliser suspension. For further information on the withdrawal of corticosteroids, when tapering off systemic corticosteroids some patients will experience steroid withdrawal symptoms, e.g. joint and/or muscle pain, lack of energy and depression or even a decreased lung function. Such patients must be advised to continue the inhaled budesonide therapy, but they should also be examined for any objective signs of adrenocortical insufficiency. If such signs are present, the dose of the systemic corticosteroid should be temporarily increased and then tapered off even more slowly. In periods of stress or severe asthma attacks, patients in the transition phase may require treatment with systemic corticosteroids.
Pseudocroup: In infants and children with pseudocroup, the commonly used dose is 2 mg of nebulised budesonide. This is given as a single administration, or as two 1 mg doses separated by 30 minutes. Dosing can be repeated every 12 hours for a maximum of 36 hours or until clinical improvement.
* চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Administration
Should be mixed with 0.9 % saline to a volume of 2 ml.
Division of the dose and miscibility: The contents of the single-dose container may be divided for adjustment of the dose. Half the ampoule contents should be placed in the Nebuliser cup and mixed with an equal volume of 0.9% sodium chloride solution. To ensure accurate dosing the use of a measuring syringe is recommended. Budesonide Nebuliser Suspension may be mixed with 0.9% sodium chloride solution and with solutions for inhalation containing terbutaline, salbutamol, sodium cromoglycate or ipratropium.
Nebuliser: Budesonide Nebuliser Suspension must be administered with a jet Nebuliser supplied with a mouthpiece or mask. The Nebuliser should be connected to an air compressor with adequate air flow (5-8 l/min), and the filling volume should be 2-4 ml. There can be variation in the performance (dose delivered) between nebulizers, even those of the same make and model.
Instruction for use-
Division of the dose and miscibility: The contents of the single-dose container may be divided for adjustment of the dose. Half the ampoule contents should be placed in the Nebuliser cup and mixed with an equal volume of 0.9% sodium chloride solution. To ensure accurate dosing the use of a measuring syringe is recommended. Budesonide Nebuliser Suspension may be mixed with 0.9% sodium chloride solution and with solutions for inhalation containing terbutaline, salbutamol, sodium cromoglycate or ipratropium.
Nebuliser: Budesonide Nebuliser Suspension must be administered with a jet Nebuliser supplied with a mouthpiece or mask. The Nebuliser should be connected to an air compressor with adequate air flow (5-8 l/min), and the filling volume should be 2-4 ml. There can be variation in the performance (dose delivered) between nebulizers, even those of the same make and model.
Instruction for use-
- The spray container should be shaken before use.
- To minimise the risk of oropharyngeal candida infection, the patient should rinse their mouth out with water after inhaling.
- To prevent irritation of the facial skin the face should be washed after using the Nebuliser with a mask.
- The Nebuliser should be cleaned after each use.
- Wash the Nebuliser container and mouthpiece or face-mask in warm water using a mild detergent in accordance with the manufacturer’s instructions. Rinse well and dry it by connecting the Nebuliser container to the compressor or the air inlet.
* চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Interaction
Budetide Nebuliser Suspension can increase the efficacy of inhaled beta-2-sympathomimetics.
Co-treatment with CYP3A inhibitors, including cobicistat-containing products, is expected to increase the risk of systemic side-effects. The combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side-effects, in which case patients should be monitored for systemic corticosteroid side-effects.
The metabolism of Budetide is primarily mediated by CYP3A4. Inhibitors of this enzyme, e.g., ketoconazole and itraconazole, can therefore increase systemic exposure to Budetide several times. Since there are no data to support dosage recommendations, the combination should be avoided. If this is not possible, the period between treatments should be as long as possible and a reduction of the Budetide dose could also be considered. Limited data about this interaction for high-dose inhaled Budetide indicate that marked increases in plasma levels (on average four- fold) may occur if itraconazole, 200 mg once daily, is administered concomitantly with inhaled Budetide (single dose of 1000 µg).
Other potent CYP3A4 inhibitors such as erythromycin, clarithromycin, ritonavir and saquinavir are also likely to markedly increase plasma concentrations of Budetide.
Cimetidine has a weak but clinically insignificant inhibiting effect on hepatic metabolism of Budetide.
Raised plasma concentrations of and enhanced effects of corticosteroids have been observed in women also treated with oestrogens and contraceptive steroids, but no effect has been observed with Budetide and concomitant intake of low dose combination oral contraceptives.
The suppressive effect on adrenal function is additive if used concomitantly with systemic or intranasal steroids.
Because adrenal function may be suppressed, an ACTH stimulation test for diagnosing pituitary insufficiency might show false results (low values).
Co-treatment with CYP3A inhibitors, including cobicistat-containing products, is expected to increase the risk of systemic side-effects. The combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side-effects, in which case patients should be monitored for systemic corticosteroid side-effects.
The metabolism of Budetide is primarily mediated by CYP3A4. Inhibitors of this enzyme, e.g., ketoconazole and itraconazole, can therefore increase systemic exposure to Budetide several times. Since there are no data to support dosage recommendations, the combination should be avoided. If this is not possible, the period between treatments should be as long as possible and a reduction of the Budetide dose could also be considered. Limited data about this interaction for high-dose inhaled Budetide indicate that marked increases in plasma levels (on average four- fold) may occur if itraconazole, 200 mg once daily, is administered concomitantly with inhaled Budetide (single dose of 1000 µg).
Other potent CYP3A4 inhibitors such as erythromycin, clarithromycin, ritonavir and saquinavir are also likely to markedly increase plasma concentrations of Budetide.
Cimetidine has a weak but clinically insignificant inhibiting effect on hepatic metabolism of Budetide.
Raised plasma concentrations of and enhanced effects of corticosteroids have been observed in women also treated with oestrogens and contraceptive steroids, but no effect has been observed with Budetide and concomitant intake of low dose combination oral contraceptives.
The suppressive effect on adrenal function is additive if used concomitantly with systemic or intranasal steroids.
Because adrenal function may be suppressed, an ACTH stimulation test for diagnosing pituitary insufficiency might show false results (low values).
Contraindications
Budesonide is not indicated for the treatment of acute dyspnoea or status asthmaticus. These conditions should be treated with short acting β-sympathomimetics and other bronchodilators.
Side Effects
The following definitions apply to the incidence of undesirable effects: Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data).
Facial irritation, as an example of a hypersensitivity reaction, has occurred in some cases when a Nebuliser with a face mask has been used. To prevent irritation the facial skin should be washed with water after use of the face mask.
In-placebo controlled studies, cataract was also uncommonly reported in the placebo group.
Clinical trials with 13,119 patients on inhaled Budetide and 7,278 patients on placebo have been pooled. The frequency of anxiety was 0.52% on inhaled Budetide and 0.63% on placebo; that of depression was 0.67% on inhaled Budetide and 1.15% on placebo.
There is an increased risk of pneumonia in patients with newly diagnosed COPD starting treatment with inhaled corticosteroids. However a weighted assessment of 8 pooled clinical trials involving 4643 COPD patients treated with Budetide and 3,643 patients randomized to non-ICS treatments did not demonstrate an increased risk for pneumonia. The results from the first 7 of these 8 trials have been published as a meta-analysis.
Treatment with inhaled Budetide may result in candida infection in the oropharynx. Experience has shown that candida infection occurs less often when inhalation is performed before meals and/or when the mouth is rinsed after inhalation. In most cases this condition responds to topical anti-fungal therapy without discontinuing treatment with inhaled Budetide.
Coughing can usually be prevented by inhaling a beta-2 agonist (e.g. terbutaline) 5-10 minutes before administration of Budetide 0.5mg Nebuliser Suspension.
Occasionally, signs or symptoms of systemic glucocorticosteroid-side effects may occur with inhaled glucocorticosteroids, probably depending on dose, exposure time, concomitant and previous corticosteroid exposure, and individual sensitivity. These may include adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, cataract and glaucoma, and susceptibility to infections. The ability to adapt to stress may be impaired. The systemic effects described, however, are much less likely to occur with inhaled Budetide than with oral corticosteroids.
Facial irritation, as an example of a hypersensitivity reaction, has occurred in some cases when a Nebuliser with a face mask has been used. To prevent irritation the facial skin should be washed with water after use of the face mask.
In-placebo controlled studies, cataract was also uncommonly reported in the placebo group.
Clinical trials with 13,119 patients on inhaled Budetide and 7,278 patients on placebo have been pooled. The frequency of anxiety was 0.52% on inhaled Budetide and 0.63% on placebo; that of depression was 0.67% on inhaled Budetide and 1.15% on placebo.
There is an increased risk of pneumonia in patients with newly diagnosed COPD starting treatment with inhaled corticosteroids. However a weighted assessment of 8 pooled clinical trials involving 4643 COPD patients treated with Budetide and 3,643 patients randomized to non-ICS treatments did not demonstrate an increased risk for pneumonia. The results from the first 7 of these 8 trials have been published as a meta-analysis.
Treatment with inhaled Budetide may result in candida infection in the oropharynx. Experience has shown that candida infection occurs less often when inhalation is performed before meals and/or when the mouth is rinsed after inhalation. In most cases this condition responds to topical anti-fungal therapy without discontinuing treatment with inhaled Budetide.
Coughing can usually be prevented by inhaling a beta-2 agonist (e.g. terbutaline) 5-10 minutes before administration of Budetide 0.5mg Nebuliser Suspension.
Occasionally, signs or symptoms of systemic glucocorticosteroid-side effects may occur with inhaled glucocorticosteroids, probably depending on dose, exposure time, concomitant and previous corticosteroid exposure, and individual sensitivity. These may include adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, cataract and glaucoma, and susceptibility to infections. The ability to adapt to stress may be impaired. The systemic effects described, however, are much less likely to occur with inhaled Budetide than with oral corticosteroids.
Pregnancy & Lactation
Pregnancy: There are no adequate and well-controlled studies in pregnant women. It is important for both foetus and mother to maintain an adequate asthma treatment during pregnancy. As with other drugs administered during pregnancy, the benefit of the administration of budesonide for the mother should be weighed against the risks to the foetus.
Breastfeeding: Budesonide is excreted in breast milk. However, at therapeutic doses of budesonide no effects on the suckling child are anticipated. Budesonide can be used during breast feeding. Maintenance treatment with inhaled budesonide (200 or 400 micrograms twice daily) in asthmatic nursing women results in negligible systemic exposure to budesonide in breast-fed infants. In a pharmacokinetic study, the estimated daily infant dose was 0.3% of the daily maternal dose for both dose levels, and the average plasma concentration in infants was estimated to be 1/600th of the concentrations observed in maternal plasma, assuming complete infant oral bioavailability. Budesonide concentrations in infant plasma samples were all less than the limit of quantification. Based on data from inhaled budesonide and the fact that budesonide exhibits linear PK properties within the therapeutic dosage intervals after nasal, inhaled, oral and rectal administrations, at therapeutic doses of budesonide, exposure to the suckling child is anticipated to be low.
Breastfeeding: Budesonide is excreted in breast milk. However, at therapeutic doses of budesonide no effects on the suckling child are anticipated. Budesonide can be used during breast feeding. Maintenance treatment with inhaled budesonide (200 or 400 micrograms twice daily) in asthmatic nursing women results in negligible systemic exposure to budesonide in breast-fed infants. In a pharmacokinetic study, the estimated daily infant dose was 0.3% of the daily maternal dose for both dose levels, and the average plasma concentration in infants was estimated to be 1/600th of the concentrations observed in maternal plasma, assuming complete infant oral bioavailability. Budesonide concentrations in infant plasma samples were all less than the limit of quantification. Based on data from inhaled budesonide and the fact that budesonide exhibits linear PK properties within the therapeutic dosage intervals after nasal, inhaled, oral and rectal administrations, at therapeutic doses of budesonide, exposure to the suckling child is anticipated to be low.
Precautions & Warnings
The transfer of patients treated with oral corticosteroids to the inhaled corticosteroid and their subsequent management requires special care. The patients should be in a reasonably stable state before initiating a high dose of inhaled corticosteroid in addition to their usual maintenance dose of systemic corticosteroid. After about 10 days, withdrawal of the systemic corticosteroid is started by reducing the daily dose gradually (by for example 2.5 milligrams prednisolone or the equivalent each month) to the lowest possible level. It may be possible to completely replace the oral corticosteroid with inhaled corticosteroid. Transferred patients whose adrenocortical function is impaired may need supplementary systemic corticosteroid during periods of stress e.g. surgery, infection or worsening asthma attacks.
Patients who have required high dose emergency corticosteroid therapy or prolonged treatment at the highest recommended dose of inhaled corticosteroids, may also be at risk of impaired adrenal function. These patients may exhibit signs and symptoms of adrenal insufficiency when exposed to severe stress. Additional systemic corticosteroid treatment should be considered during periods of stress or elective surgery.
During transfer from oral therapy to inhaled Budetide, symptoms may appear that had previously been suppressed by systemic treatment with glucocorticosteroids, for example symptoms of allergic rhinitis, eczema, muscle and joint pain. Specific treatment should be co-administered to treat these conditions.
Some patients may feel unwell in a non-specific way during the withdrawal of systemic corticosteroids despite maintenance or even improvement in respiratory function. Such patients should be encouraged to continue treatment with inhaled Budetide and withdrawal of oral corticosteroid unless there are clinical signs to indicate the contrary, for example signs which might indicate adrenal insufficiency.
As with other inhalation therapies paradoxical bronchospasm may occur, manifested by an immediate increase in wheezing and shortness of breath after dosing. Paradoxical bronchospasm responds to a rapid-acting inhaled bronchodilator and should be treated straight away. Budetide should be discontinued immediately, the patient should be assessed and, if necessary, alternative treatment instituted.
When an acute episode of dyspnoea occurs despite a well monitored treatment, a rapid-acting inhaled bronchodilator should be used and medical reassessment should be considered. If despite maximum doses of inhaled corticosteroids, asthma symptoms are not adequately controlled, patients may require short-term treatment with systemic corticosteroids. In such cases, it is necessary to maintain the inhaled corticosteroid therapy in association with treatment by the systemic route.
Systemic effects may occur with any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur with inhalation treatment than with oral corticosteroids.
Possible systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). It is important, therefore, that the dose of inhaled corticosteroid is titrated to the lowest dose at which effective control of asthma is maintained.
Influence on growth: It is recommended that the height of children receiving prolonged treatment with inhaled corticosteroids is regularly monitored. If growth is slowed, therapy should be re-evaluated with the aim of reducing the dose of inhaled corticosteroid. The benefits of the corticosteroid therapy and the possible risks of growth suppression must be carefully weighed. In addition, consideration should be given to referring the patient to a paediatric respiratory specialist.
Patients who have previously been dependent on oral corticosteroids may, as a result of prolonged systemic corticosteroid therapy, experience effects of impaired adrenal function. Recovery may take a considerable amount of time after cessation of oral corticosteroid therapy and hence oral steroid-dependent patients transferred to Budetide may remain at risk from impaired adrenocortical function for some considerable time. In such circumstances hypothalamic pituitary adrenocortical (HPA) axis function should be monitored regularly.
Oral candidiasis may occur during the therapy with inhaled corticosteroids. This infection may require treatment with appropriate antifungal therapy and in some patients discontinuation of treatment may be necessary
Exacerbation of clinical symptoms of asthma may be due to acute respiratory tract bacterial infections and treatment with appropriate antibiotics may be required. Such patients may need to increase the dose of inhaled Budetide and a short course of oral corticosteroids may be required. A rapid-acting inhaled bronchodilator should be used as “rescue” medication to relieve acute asthma symptoms.
Special care and adequate specific therapeutic control of patients with active and quiescent pulmonary tuberculosis is necessary before commencing treatment with inhaled Budetide. Similarly patients with fungal, viral or other infections of the airways require close observation and special care and should use Budetide only if they are also receiving adequate treatment for such infections.
In patients with excessive mucous secretion in the respiratory tract, short-term therapy with oral corticosteroids may be necessary.
In patients with severe hepatic dysfunction, treatment with inhaled Budetide can result in a reduced elimination rate and hence enhanced systemic availability. Possible systemic effects may then result and therefore HPA axis function in these patients should be monitored at regular intervals.
Concomitant treatment with ketoconazole, HIV protease inhibitors or other potent CYP3A4 inhibitors should be avoided. If this is not possible the time interval between administration of the two drugs should be as long as possible.
Recent epidemiological studies show that there is an increased incidence of pneumonia in patients with Chronic Obstructive Pulmonary Disease (COPD) treated with inhaled corticosteroids, with an adjusted odds ratio of 1.7. Care should be exercised in prescribing Budetide for those patients whose respiratory disease might have a component of COPD.
Visual disturbance: Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Budetide Nebuliser Suspension should be used with a jet Nebuliser device. An ultrasonic Nebuliser should not be used as this is not appropriate for Nebuliser suspensions.
Patients who have required high dose emergency corticosteroid therapy or prolonged treatment at the highest recommended dose of inhaled corticosteroids, may also be at risk of impaired adrenal function. These patients may exhibit signs and symptoms of adrenal insufficiency when exposed to severe stress. Additional systemic corticosteroid treatment should be considered during periods of stress or elective surgery.
During transfer from oral therapy to inhaled Budetide, symptoms may appear that had previously been suppressed by systemic treatment with glucocorticosteroids, for example symptoms of allergic rhinitis, eczema, muscle and joint pain. Specific treatment should be co-administered to treat these conditions.
Some patients may feel unwell in a non-specific way during the withdrawal of systemic corticosteroids despite maintenance or even improvement in respiratory function. Such patients should be encouraged to continue treatment with inhaled Budetide and withdrawal of oral corticosteroid unless there are clinical signs to indicate the contrary, for example signs which might indicate adrenal insufficiency.
As with other inhalation therapies paradoxical bronchospasm may occur, manifested by an immediate increase in wheezing and shortness of breath after dosing. Paradoxical bronchospasm responds to a rapid-acting inhaled bronchodilator and should be treated straight away. Budetide should be discontinued immediately, the patient should be assessed and, if necessary, alternative treatment instituted.
When an acute episode of dyspnoea occurs despite a well monitored treatment, a rapid-acting inhaled bronchodilator should be used and medical reassessment should be considered. If despite maximum doses of inhaled corticosteroids, asthma symptoms are not adequately controlled, patients may require short-term treatment with systemic corticosteroids. In such cases, it is necessary to maintain the inhaled corticosteroid therapy in association with treatment by the systemic route.
Systemic effects may occur with any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur with inhalation treatment than with oral corticosteroids.
Possible systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decrease in bone mineral density, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). It is important, therefore, that the dose of inhaled corticosteroid is titrated to the lowest dose at which effective control of asthma is maintained.
Influence on growth: It is recommended that the height of children receiving prolonged treatment with inhaled corticosteroids is regularly monitored. If growth is slowed, therapy should be re-evaluated with the aim of reducing the dose of inhaled corticosteroid. The benefits of the corticosteroid therapy and the possible risks of growth suppression must be carefully weighed. In addition, consideration should be given to referring the patient to a paediatric respiratory specialist.
Patients who have previously been dependent on oral corticosteroids may, as a result of prolonged systemic corticosteroid therapy, experience effects of impaired adrenal function. Recovery may take a considerable amount of time after cessation of oral corticosteroid therapy and hence oral steroid-dependent patients transferred to Budetide may remain at risk from impaired adrenocortical function for some considerable time. In such circumstances hypothalamic pituitary adrenocortical (HPA) axis function should be monitored regularly.
Oral candidiasis may occur during the therapy with inhaled corticosteroids. This infection may require treatment with appropriate antifungal therapy and in some patients discontinuation of treatment may be necessary
Exacerbation of clinical symptoms of asthma may be due to acute respiratory tract bacterial infections and treatment with appropriate antibiotics may be required. Such patients may need to increase the dose of inhaled Budetide and a short course of oral corticosteroids may be required. A rapid-acting inhaled bronchodilator should be used as “rescue” medication to relieve acute asthma symptoms.
Special care and adequate specific therapeutic control of patients with active and quiescent pulmonary tuberculosis is necessary before commencing treatment with inhaled Budetide. Similarly patients with fungal, viral or other infections of the airways require close observation and special care and should use Budetide only if they are also receiving adequate treatment for such infections.
In patients with excessive mucous secretion in the respiratory tract, short-term therapy with oral corticosteroids may be necessary.
In patients with severe hepatic dysfunction, treatment with inhaled Budetide can result in a reduced elimination rate and hence enhanced systemic availability. Possible systemic effects may then result and therefore HPA axis function in these patients should be monitored at regular intervals.
Concomitant treatment with ketoconazole, HIV protease inhibitors or other potent CYP3A4 inhibitors should be avoided. If this is not possible the time interval between administration of the two drugs should be as long as possible.
Recent epidemiological studies show that there is an increased incidence of pneumonia in patients with Chronic Obstructive Pulmonary Disease (COPD) treated with inhaled corticosteroids, with an adjusted odds ratio of 1.7. Care should be exercised in prescribing Budetide for those patients whose respiratory disease might have a component of COPD.
Visual disturbance: Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Budetide Nebuliser Suspension should be used with a jet Nebuliser device. An ultrasonic Nebuliser should not be used as this is not appropriate for Nebuliser suspensions.
Use in Special Populations
Pediatric population: Due to the risk of growth retardation in the pediatric population, growth should be monitored.
Overdose Effects
Symptoms: Acute overdose with Budetide usually does not constitute a clinical problem. The only harmful effect after a large amount of sprays during a short period is a suppression of the cortex function. If it is a matter of chronic use of very high doses, effects such as a degree of cortex atrophy in addition to adrenocortical suppression may occur.
Acute overdosage: There is no need for acute measures. The treatment with Budetide should be continued with the lowest possible effective maintenance dose, and the adrenocortical function will repair itself automatically within 1-2 days.
Chronic overdosage: The patient should be treated as a steroid dependent and be transferred to a suitable maintenance dose with a systemic steroid, for example prednisolone. When the condition is stabilized, the patient should continue the treatment with the inhalation of Budetide at the recommended dose.
Acute overdosage: There is no need for acute measures. The treatment with Budetide should be continued with the lowest possible effective maintenance dose, and the adrenocortical function will repair itself automatically within 1-2 days.
Chronic overdosage: The patient should be treated as a steroid dependent and be transferred to a suitable maintenance dose with a systemic steroid, for example prednisolone. When the condition is stabilized, the patient should continue the treatment with the inhalation of Budetide at the recommended dose.
Therapeutic Class
Nasal Decongestants & Other Nasal Preparations, Respiratory corticosteroids
Storage Conditions
Keep below 30°C temperature, away from light & moisture. Keep out of the reach of children.