Unit Price:
৳ 100.00
(5 x 6: ৳ 3,000.00)
Strip Price:
৳ 600.00
Also available as:
Indications
Chelova is indicated for the treatment of chronic iron overload due to repeated blood transfusions in adult and pediatric patients aged 2 years and over with thalassaemia, sickle cell disease and myelodysplastic syndrome. Also indicated for treating chronic iron overload in patients with non-transfusion-dependent thalassemia syndromes aged 10 years and older.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Pharmacology
Deferasirox is an orally active chelator that is highly selective for iron, It is a tridentate ligand that binds iron with high affinity in a 2:1 ratio. Deferasirox promotes excretion of iron, primarily in the faeces. Deferasirox has low affinity for zinc and copper, and does not cause constant low serum levels of these metals. In an iron balance metabolic study in iron overloaded adult thalassaemia patients, Deferasirox at daily doses of 10, 20 and 40 mg/kg induced the mean net excretion of 0.119, 0.329, and 0,445 mg Fe/kg body weight/day, respectively.
Dosage
The drug should be started after the transfusion of approximately 20 units (about 100 ml/kg) of packed red blood cells or evidence of chronic iron overload is present (e.g. serum ferritin >1,000 microgram/L).
The recommended initial daily dose of Deferasirox is 20-40 mg/kg body weight per day in empty stomach preferably half an hour before breakfast. The tablets are dispersed by stirring in a glass of water or apple or orange juice (100 to 200 ml) until a fine suspension is obtained. After the suspension has been swallowed, any residue must be re-suspended in a small volume of water or juice and swallowed, The tablets must not be chewed or swallowed whole. Dispersion in carbonated drinks or milk is not recommended due to foaming and slow dispersion, respectively.
Serum ferritin to be monitored every month and the dose is adjusted accordingly every 3 to 6 months based on the trends in serum ferritin. If iron overload is inadequately controlled, doses of up to 40 mg/kg may be considered. If serum ferritin level is controlled, dose reductions in steps of 5 to 10 mg/kg should be considered to maintain serum ferritin at 500 microgram/l. Dose interruption can be done if sustained control is achieved,
The recommended initial daily dose of Deferasirox is 20-40 mg/kg body weight per day in empty stomach preferably half an hour before breakfast. The tablets are dispersed by stirring in a glass of water or apple or orange juice (100 to 200 ml) until a fine suspension is obtained. After the suspension has been swallowed, any residue must be re-suspended in a small volume of water or juice and swallowed, The tablets must not be chewed or swallowed whole. Dispersion in carbonated drinks or milk is not recommended due to foaming and slow dispersion, respectively.
Serum ferritin to be monitored every month and the dose is adjusted accordingly every 3 to 6 months based on the trends in serum ferritin. If iron overload is inadequately controlled, doses of up to 40 mg/kg may be considered. If serum ferritin level is controlled, dose reductions in steps of 5 to 10 mg/kg should be considered to maintain serum ferritin at 500 microgram/l. Dose interruption can be done if sustained control is achieved,
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Administration
Deferasirox must be taken once daily on an empty stomach at least 30 minutes before food, preferably at the same time each day. The tablets are dispersed by stirring in a glass of water or apple or orange juice (100 to 200 mL) until a line suspension is obtained. After the suspension has been swallowed, any residue must be resuspended in a small volume of water or juice and swallowed. The tablets must not be chewed or swallowed whole. Dispersion in carbonated drinks or milk is not recommended due to foaming and slow dispersion, respectively.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Interaction
Should not be taken with aluminium-containing antacids, Caution to be taken when combined with drugs metabolized through CYP3A4 (e.g. cyclosporine, simvastatin, hormonal contraceptive, midazolam). Dose increase should be considered when concomitantly used with rifampicin, phenytoin, phenobarbital, ritonavir etc. Interaction with other CYP2C8 substrates like paclitaxel cannot be excluded. Caution to be taken when combined with drugs with ulcerogenic potential (e.g. NSAIDS, corticosteroids, oral bisphosphonates) or with anticoagulants.
Contraindications
Deferasirox is contraindicated in hypersensitivity to the active substance or to any of the excipients. Cannot be given when creatinine clearance is less than 40 ml/min or serum creatinine >2 times the upper limit of normal.
Side Effects
Cytopenias may occur diarrhea, constipation, vomiting, nausea, abdominal pain, abdominal distension, dyspepsia upper gastrointestinal ulceration and hemorrhage have been reported. Headache, dizziness and main
CNS involvement. Elevation of hepatic transaminases may occur. Cases of Stevens-Johnson syndrome (SJS) and skin rash have been rarely reported. Auditory and ocular disturbances may occur.
CNS involvement. Elevation of hepatic transaminases may occur. Cases of Stevens-Johnson syndrome (SJS) and skin rash have been rarely reported. Auditory and ocular disturbances may occur.
Pregnancy & Lactation
Pregnancy Category C. There are no adequate and well-controlled studies with Deferasirox in pregnant women. Administration of Deferasirox to animals during pregnancy and lactation resulted in decreased offspring viability and an increase in renal anomalies in male offspring at exposures that were less than the recommended human exposure. Deferasirox should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is not known whether Deferasirox is excreted in human milk.
Precautions & Warnings
Fatal and non-fatal gastrointestinal bleeding, ulceration, and irritation may occur during Chelova treatment , Use caution in patients who are taking Chelova in combination with drugs that have known ulcerogenic or hemorrhagic potential, such as NSAIDs, corticosteroids, oral bisphosphonates, and anticoagulants.
Cytopenias, including agranulocytosis, neutropenia and thrombocytopenia have been reported. Monitor blood counts during Chelova therapy.
Serious hypersensitivity reactions have been reported, If reactions are severe, discontinue Chelova and institute appropriate medical intervention.
Cytopenias, including agranulocytosis, neutropenia and thrombocytopenia have been reported. Monitor blood counts during Chelova therapy.
Serious hypersensitivity reactions have been reported, If reactions are severe, discontinue Chelova and institute appropriate medical intervention.
Use in Special Populations
Pediatric use: The safety and efficacy of Chelova in pediatric patients was similar to that of adult patients,
and younger pediatric patients responded similarly to older pediatric patients. The recommended starting dose and dosing modification are the same for children and adults.
Geriatric use: Elderly patients are at increased risk for Chelova toxicity due to the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Renal impairment: Chelova has not been studied in patients with renal impairment.
Hepatic impairment: Chelova has not been studied in patients with hepatic impairment.
and younger pediatric patients responded similarly to older pediatric patients. The recommended starting dose and dosing modification are the same for children and adults.
Geriatric use: Elderly patients are at increased risk for Chelova toxicity due to the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Renal impairment: Chelova has not been studied in patients with renal impairment.
Hepatic impairment: Chelova has not been studied in patients with hepatic impairment.
Overdose Effects
The maximal dose of Chelova is 40 mg/kg body weight. There is no information about overdose of Chelova.
Therapeutic Class
Antidote preparations
Storage Conditions
Store in a dry place below 25°C, and protect from light. Keep out of the reach of children.
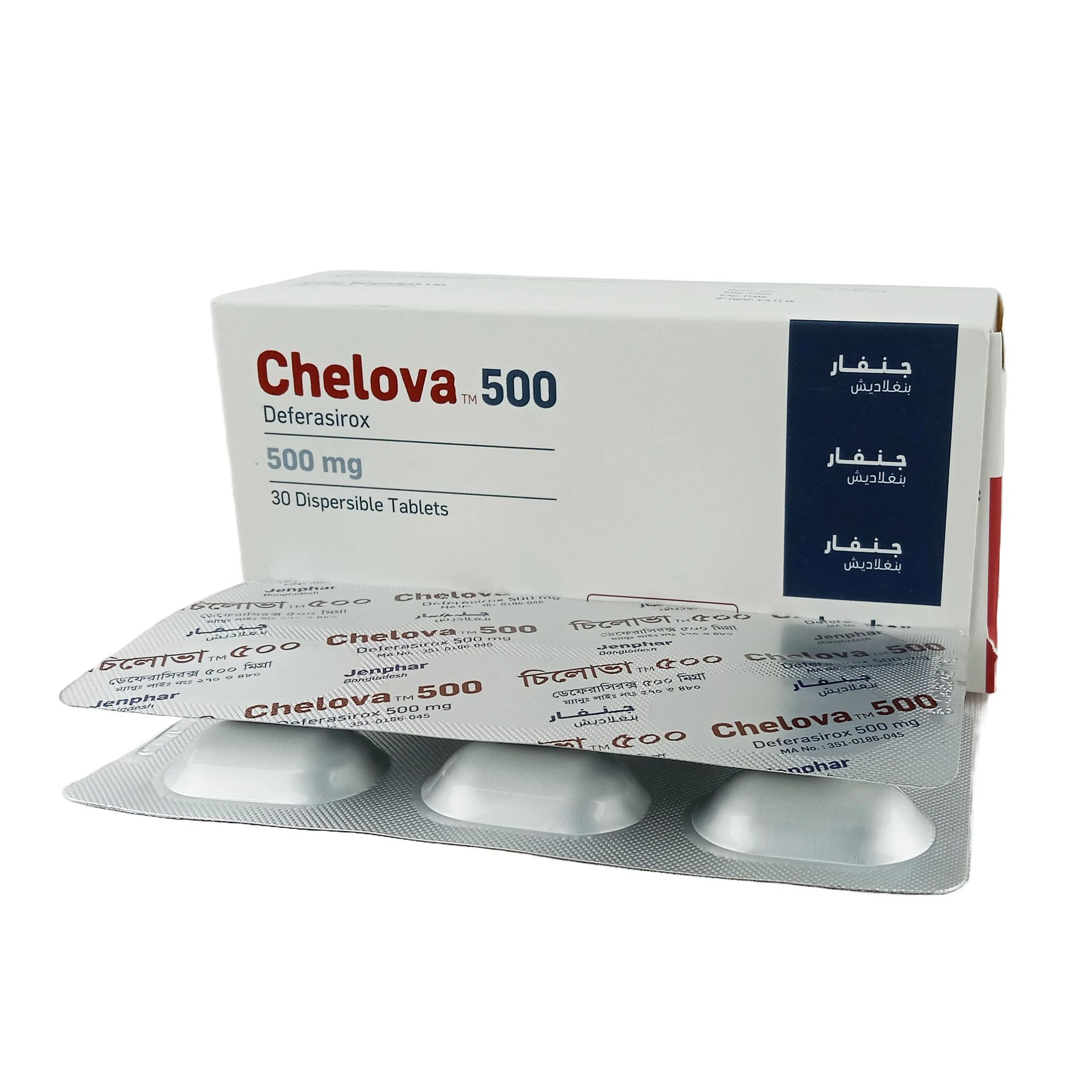
Pack Images: Chelova 500 mg Tablet