Unit Price:
৳ 1,660.00
(1 x 30: ৳ 49,800.00)
Strip Price:
৳ 49,800.00
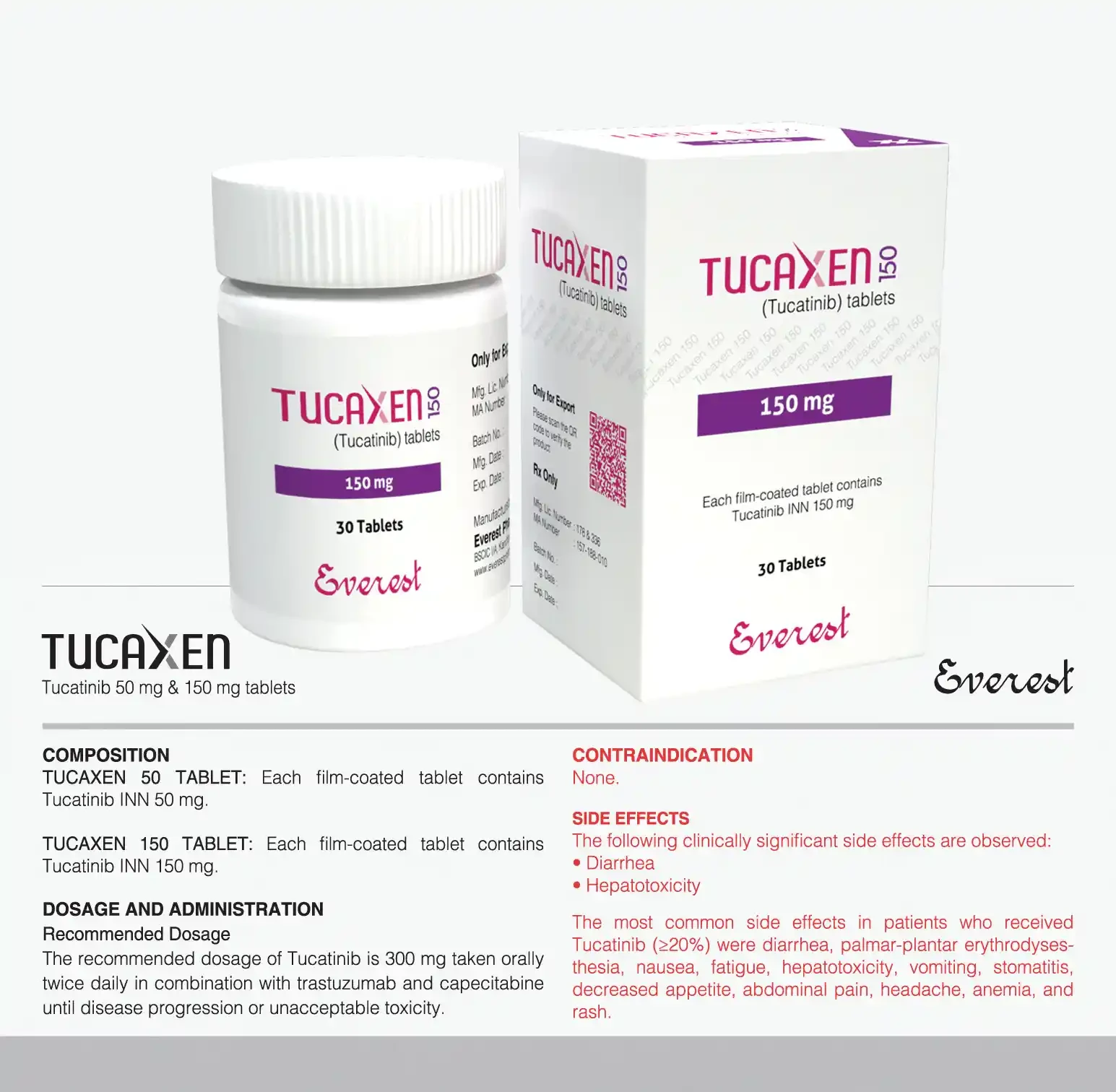
Indications
Tucaxen is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Pharmacology
Tucatinib is a tyrosine kinase inhibitor of HER2. In vitro, tucatinib inhibits phosphorylation of HER2 and HER3, resulting in inhibition of downstream MAPK and AKT signaling and cell proliferation, and showed anti-tumor activity in HER2 expressing tumor cells. In vivo, tucatinib inhibited the growth of HER2 expressing tumors. The combination of tucatinib and trastuzumab showed increased anti-tumor activity in vitro and in vivo compared to either drug alone.
Exposure Response Relationship: Tucatinib exposure-response relationships and the time course of pharmacodynamics response have not been fully characterized.
Cardiac Electrophysiology: No large mean increase in QTc (i.e., > 20 ms) was detected following treatment with Tucatinib at the recommended dose of 300 mg taken orally twice daily.
Pharmacokinetics: Tucatinib AUC0-INF and Cmax increases proportionally over a dosage range from 50 mg to 300 mg (0.17 to 1 times the approved recommended dosage). Tucatinib exhibited 1.7-fold accumulation for AUC and 1.5-fold accumulation for Cmax following administration of Tucatinib 300 mg twice daily for 14 days. Time to steady state was approximately 4 days.
Absorption: The median time to peak plasma concentration of Tucatinib was approximately 2 hours (range 1 to 4 hours).
Distribution: The geometric mean (CV%) apparent volume of distribution of tucatinib was approximately 1670 L (66%). The plasma protein binding was 97.1% at clinically relevant concentrations.
Elimination: The geometric mean (CV%) half-life of tucatinib was approximately 8.5 (21%) hours and apparent clearance was 148 L/h (55%).
Metabolism: Tucatinib is metabolized primarily by CYP2C8 and to a lesser extent via CYP3A.
Excretion: Following a single oral dose of 300 mg radiolabeled Tucatinib, approximately 86% of the total radiolabeled dose was recovered in feces (16% of the administered dose as unchanged tucatinib) and 4.1% in urine with an overall total recovery of 90% within 13 days post-dose. In plasma, approximately 76% of the plasma radioactivity was unchanged, 19% was attributed to identified metabolites, and approximately 5% was unassigned.
Exposure Response Relationship: Tucatinib exposure-response relationships and the time course of pharmacodynamics response have not been fully characterized.
Cardiac Electrophysiology: No large mean increase in QTc (i.e., > 20 ms) was detected following treatment with Tucatinib at the recommended dose of 300 mg taken orally twice daily.
Pharmacokinetics: Tucatinib AUC0-INF and Cmax increases proportionally over a dosage range from 50 mg to 300 mg (0.17 to 1 times the approved recommended dosage). Tucatinib exhibited 1.7-fold accumulation for AUC and 1.5-fold accumulation for Cmax following administration of Tucatinib 300 mg twice daily for 14 days. Time to steady state was approximately 4 days.
Absorption: The median time to peak plasma concentration of Tucatinib was approximately 2 hours (range 1 to 4 hours).
Distribution: The geometric mean (CV%) apparent volume of distribution of tucatinib was approximately 1670 L (66%). The plasma protein binding was 97.1% at clinically relevant concentrations.
Elimination: The geometric mean (CV%) half-life of tucatinib was approximately 8.5 (21%) hours and apparent clearance was 148 L/h (55%).
Metabolism: Tucatinib is metabolized primarily by CYP2C8 and to a lesser extent via CYP3A.
Excretion: Following a single oral dose of 300 mg radiolabeled Tucatinib, approximately 86% of the total radiolabeled dose was recovered in feces (16% of the administered dose as unchanged tucatinib) and 4.1% in urine with an overall total recovery of 90% within 13 days post-dose. In plasma, approximately 76% of the plasma radioactivity was unchanged, 19% was attributed to identified metabolites, and approximately 5% was unassigned.
Dosage & Administration
Recommended Dosage: The recommended dosage of Tucatinib is 300 mg taken orally twice daily in combination with trastuzumab and capecitabine until disease progression or unacceptable toxicity. Patients are advised to swallow Tucatinib tablets whole and not to chew, crush, or split prior to swallowing. Patients are advised not to ingest tablet if it is broken, cracked, or not otherwise intact. Patients are advised to take Tucatinib approximately 12 hours apart and at the same time each day with or without a meal. If the patient vomits or misses a dose of Tucatinib, Patients are advised to take the next dose at its usual scheduled time. When given in combination with Tucatinib, the recommended dosage of capecitabine is 1000 mg/m2 orally twice daily taken within 30 minutes after a meal . Tucatinib and capecitabine can be taken at the same time.
Dosage Modifications for Severe Hepatic Impairment: For patients with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 200 mg orally twice daily.
Dosage Modifications for Concomitant Use with Strong CYP2C8 Inhibitors: Avoid concomitant use of strong CYP2C8 inhibitors with Tucatinib. If concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally twice daily. After discontinuation of the strong CYP2C8 inhibitor for 3 elimination half-lives, resume the Tucatinib dose that was taken prior to initiating the inhibitor.
Dosage Modifications for Severe Hepatic Impairment: For patients with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 200 mg orally twice daily.
Dosage Modifications for Concomitant Use with Strong CYP2C8 Inhibitors: Avoid concomitant use of strong CYP2C8 inhibitors with Tucatinib. If concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally twice daily. After discontinuation of the strong CYP2C8 inhibitor for 3 elimination half-lives, resume the Tucatinib dose that was taken prior to initiating the inhibitor.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Side Effects
The following clinically significant side effects are observed: DiarrheA, Hepatotoxicity. The most common side effects in patients who received Tucaxen (>20%) were diarrhea, palmar-plantar erythrodysesthesia, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Pregnancy & Lactation
Pregnancy: Tucatinib is used in combination with trastuzumab and capecitabine. Based on findings in animals and its mechanism of action, Tucatinib can cause fetal harm when administered to a pregnant woman .There are no available human data on Tucatinib use in pregnant women to inform a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Lactation: Tucatinib is used in combination with trastuzumab and capecitabine. There are no data on the presence of Tucatinib or its metabolites in human or animal milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with Tucatinib and for at least 1 week after the last dose.
Lactation: Tucatinib is used in combination with trastuzumab and capecitabine. There are no data on the presence of Tucatinib or its metabolites in human or animal milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with Tucatinib and for at least 1 week after the last dose.
Precautions & Warnings
Diarrhea: Tucaxen can cause severe diarrhea including dehydration, hypotension, acute kidney injury, and death. In HER2CLIMB, 81% of patients who received Tucaxen experienced diarrhea, including 12% with Grade 3 diarrhea and 0.5% with Grade 4 diarrhea. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. The median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to dose reductions of Tucaxen in 6% of patients and discontinuation of Tucaxen in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB. If diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Based on the severity of the diarrhea, interrupt dose, then reduce dose or permanently discontinue Tucaxen.
Hepatotoxicity: Tucaxen can cause severe hepatotoxicity. In HER2CLIMB, 8% of patients who received Tucaxen had an ALT increase >5 x ULN, 6% had an AST increase >5 x ULN, and 1.5% had a bilirubin increase >3 x ULN (Grade >3). Hepatotoxicity led to dose reduction of Tucaxen in 8% of patients and discontinuation of Tucaxen in 1.5% of patients. Monitor ALT, AST, and bilirubin prior to starting Tucaxen, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then reduce dose or permanently discontinue Tucaxen.
Embryo-Fetal Toxicity: Based on findings from animal studies and its mechanism of action, Tucaxen can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of Tucaxen to pregnant rats and rabbits during organogenesis caused embryo-fetal mortality, reduced fetal weight and fetal abnormalities at maternal exposures >1.3 times the human exposure (AUC) at the recommended dose. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Tucaxen and for at least 1 week after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Tucaxen and for at least 1 week after the last dose. Tucaxen is used in combination with trastuzumab and capecitabine.
Hepatotoxicity: Tucaxen can cause severe hepatotoxicity. In HER2CLIMB, 8% of patients who received Tucaxen had an ALT increase >5 x ULN, 6% had an AST increase >5 x ULN, and 1.5% had a bilirubin increase >3 x ULN (Grade >3). Hepatotoxicity led to dose reduction of Tucaxen in 8% of patients and discontinuation of Tucaxen in 1.5% of patients. Monitor ALT, AST, and bilirubin prior to starting Tucaxen, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then reduce dose or permanently discontinue Tucaxen.
Embryo-Fetal Toxicity: Based on findings from animal studies and its mechanism of action, Tucaxen can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of Tucaxen to pregnant rats and rabbits during organogenesis caused embryo-fetal mortality, reduced fetal weight and fetal abnormalities at maternal exposures >1.3 times the human exposure (AUC) at the recommended dose. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Tucaxen and for at least 1 week after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Tucaxen and for at least 1 week after the last dose. Tucaxen is used in combination with trastuzumab and capecitabine.
Use in Special Populations
Pediatric Use: The safety and effectiveness of Tucaxen in pediatric patients have not been established.
Renal Impairment: The use of Tucaxen in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (ClCr < 30 mL/min estimated by Cockcroft-Gault Equation), because capecitabine is contraindicated in patients with severe renal impairment. No dose adjustment is recommended for patients with mild or moderate renal impairment (ClCr 30 to 89 mL/min).
Hepatic Impairment: Tucaxen exposure is increased in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of Tucaxen for patients with severe (Child-Pugh C) hepatic impairment. No dose adjustment for Tucaxen is required for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment.
Renal Impairment: The use of Tucaxen in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (ClCr < 30 mL/min estimated by Cockcroft-Gault Equation), because capecitabine is contraindicated in patients with severe renal impairment. No dose adjustment is recommended for patients with mild or moderate renal impairment (ClCr 30 to 89 mL/min).
Hepatic Impairment: Tucaxen exposure is increased in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of Tucaxen for patients with severe (Child-Pugh C) hepatic impairment. No dose adjustment for Tucaxen is required for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment.
Overdose Effects
There is no specific antidote, and the benefit of haemodialysis in the treatment of Tucaxen overdose is unknown. In the event of an overdose, treatment with Tucaxen should be withheld and general supportive measures should be applied.
Therapeutic Class
Cytotoxic Chemotherapy
Storage Conditions
Store below 25°C, in a cool and dry place. Keep away from light. Keep out of the reach of children.