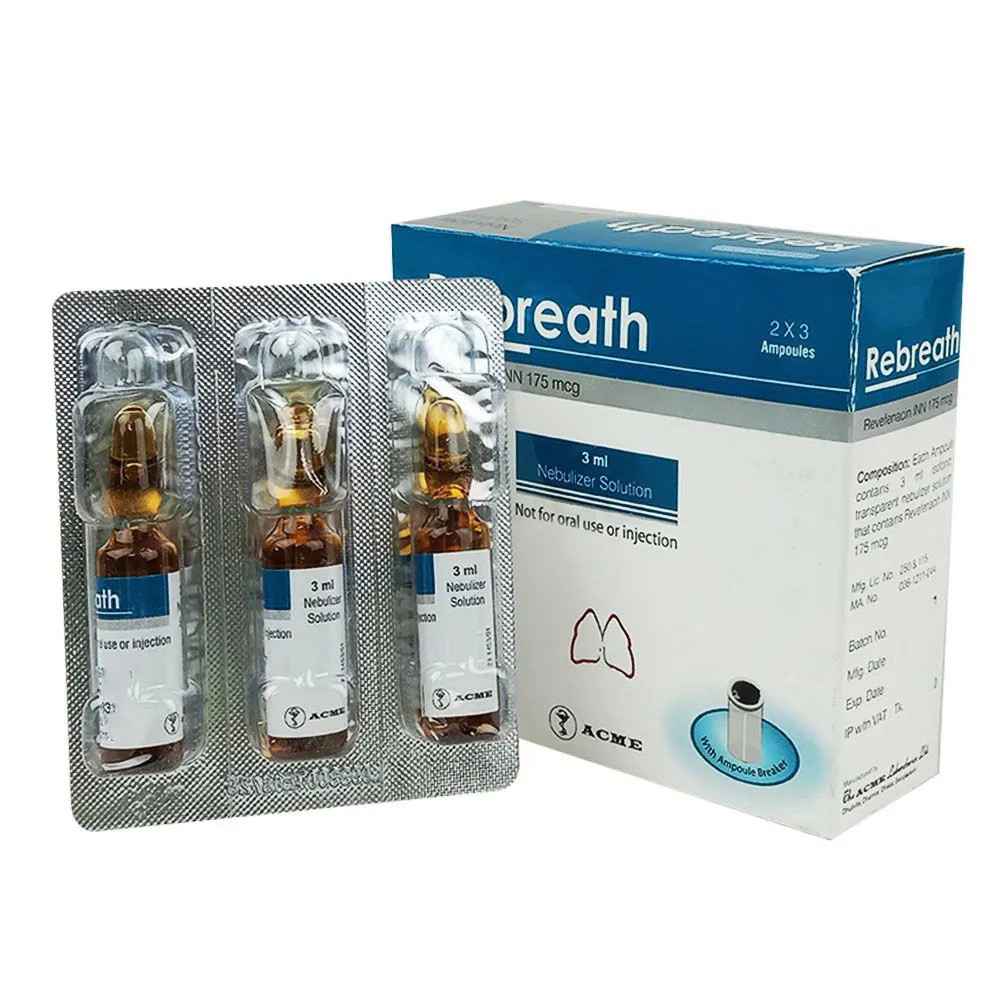
3 ml ampoule:
৳ 80.00
(2 x 3: ৳ 480.00)
Indications
Rebreath nebulizer solution is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Pharmacology
Revefenacin is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo models, prevention of methacholine- and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of revefenacin is predominantly a site-specific effect.
Dosage & Administration
The recommended dosage is 175 mcg Revefenacin (one 175 mcg unit‑dose vial) administered by oral inhalation once daily by nebulizer using a mouthpiece. Revefenacin should be administered by the orally inhaled route via a standard jet nebulizer connected to an air compressor. The safety and efficacy of Revefenacin delivered from non-compressor-based nebulizer systems have not been established. The Revefenacin unit-dose vial should only be removed from the foil pouch and opened immediately before use. The vial and any residual content should be discarded after use.
Use in Children and Adolescents: Efficacy and safety in children and adolescents under 18 years has not been established.
Geriatric Use: Based on available data, no adjustment of the dosage of Revefenacin in geriatric patients is necessary.
Renal impairment patient: No dosage adjustment is required for patients with renal impairment.
Use in Children and Adolescents: Efficacy and safety in children and adolescents under 18 years has not been established.
Geriatric Use: Based on available data, no adjustment of the dosage of Revefenacin in geriatric patients is necessary.
Renal impairment patient: No dosage adjustment is required for patients with renal impairment.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Interaction
May interact additively with concomitantly used anticholinergic medications. Avoid administration of Rebreath nebulizer solution with other anticholinergiccontaining drugs. Coadministration of Rebreath nebulizer solution with OATP1B1 and OATP1B3 inhibitors (e.g. rifampicin, cyclosporine, etc.) may lead to an increase in exposure of the active metabolite. Therefore, coadministration with Rebreath is not recommended.
Contraindications
Revefenacin is contraindicated in patients with hypersensitivity to Revefenacin or any component of this product.
Side Effects
Common side effects: Cough, runny nose, upper respiratory tract infection, headache.
Serious side effects: Glaucoma, eye pain, blurred vision, painful urination, Rash, swelling of face, mouth and tongue.
Serious side effects: Glaucoma, eye pain, blurred vision, painful urination, Rash, swelling of face, mouth and tongue.
Pregnancy & Lactation
There are no adequate and well-controlled studies with Revefenacin in pregnant women. Women should be advised to contact their physician if they become pregnant while taking Revefenacin. In animal reproduction studies, subcutaneous administration of revefenacin to pregnant rats and rabbits during the period of organogenesis produced no evidence of fetal harm at respective exposures approximately 209 times the exposure at the maximum recommended human dose (MRHD).
There is no information regarding the presence of revefenacin in human milk, the effects on the breastfed infant, or the effects on milk production. However, revefenacin was present in the milk of lactating rats following dosing during pregnancy and lactation. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Revefenacin and any potential adverse effects on the breastfed infant from Revefenacin or from the underlying maternal condition.
There is no information regarding the presence of revefenacin in human milk, the effects on the breastfed infant, or the effects on milk production. However, revefenacin was present in the milk of lactating rats following dosing during pregnancy and lactation. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Revefenacin and any potential adverse effects on the breastfed infant from Revefenacin or from the underlying maternal condition.
Precautions & Warnings
Deterioration of Disease and Acute Episodes: Rebreath should not be initiated in patients during acutely deteriorating or potentially life-threatening episodes of COPD. Rebreath has not been studied in subjects with acutely deteriorating COPD. The initiation of Rebreath in this setting is not appropriate.
Rebreath is intended as a once-daily maintenance treatment for COPD and should not be used for relief of acute symptoms, i.e. as rescue therapy for the treatment of acute episodes of bronchospasm, and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If Rebreath no longer controls symptoms of bronchoconstriction, the patient's inhaled, short-acting beta2-agonist becomes less effective, or the patient needs more inhalations of a short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of Rebreath beyond the recommended dose is not appropriate in this situation.
Paradoxical Bronchospasm: As with other inhaled medicines, Rebreath can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs following dosing with Rebreath, it should be treated immediately with an inhaled, short-acting bronchodilator; Rebreath should be discontinued immediately and alternative therapy should be instituted.
Worsening of Narrow-Angle Glaucoma: Rebreath should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g. eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
Worsening of Urinary Retention: Rebreath should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g. difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develops.
Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of Rebreath. If such a reaction occurs, therapy with Rebreath should be stopped at once and alternative treatments should be considered.
Rebreath is intended as a once-daily maintenance treatment for COPD and should not be used for relief of acute symptoms, i.e. as rescue therapy for the treatment of acute episodes of bronchospasm, and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If Rebreath no longer controls symptoms of bronchoconstriction, the patient's inhaled, short-acting beta2-agonist becomes less effective, or the patient needs more inhalations of a short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of Rebreath beyond the recommended dose is not appropriate in this situation.
Paradoxical Bronchospasm: As with other inhaled medicines, Rebreath can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs following dosing with Rebreath, it should be treated immediately with an inhaled, short-acting bronchodilator; Rebreath should be discontinued immediately and alternative therapy should be instituted.
Worsening of Narrow-Angle Glaucoma: Rebreath should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g. eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
Worsening of Urinary Retention: Rebreath should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g. difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develops.
Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of Rebreath. If such a reaction occurs, therapy with Rebreath should be stopped at once and alternative treatments should be considered.
Overdose Effects
An overdose of Rebreath may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances, or reddening of the eye), fatigue or difficulties in voiding.
Therapeutic Class
Anticholinergic bronchodilators
Storage Conditions
Store below 30°C temperature & in dry place. Protect from direct sunlight and temperature. Keep all medicines out of reach of children. Discard ampoule and other part after use. Not for oral administration or injection.