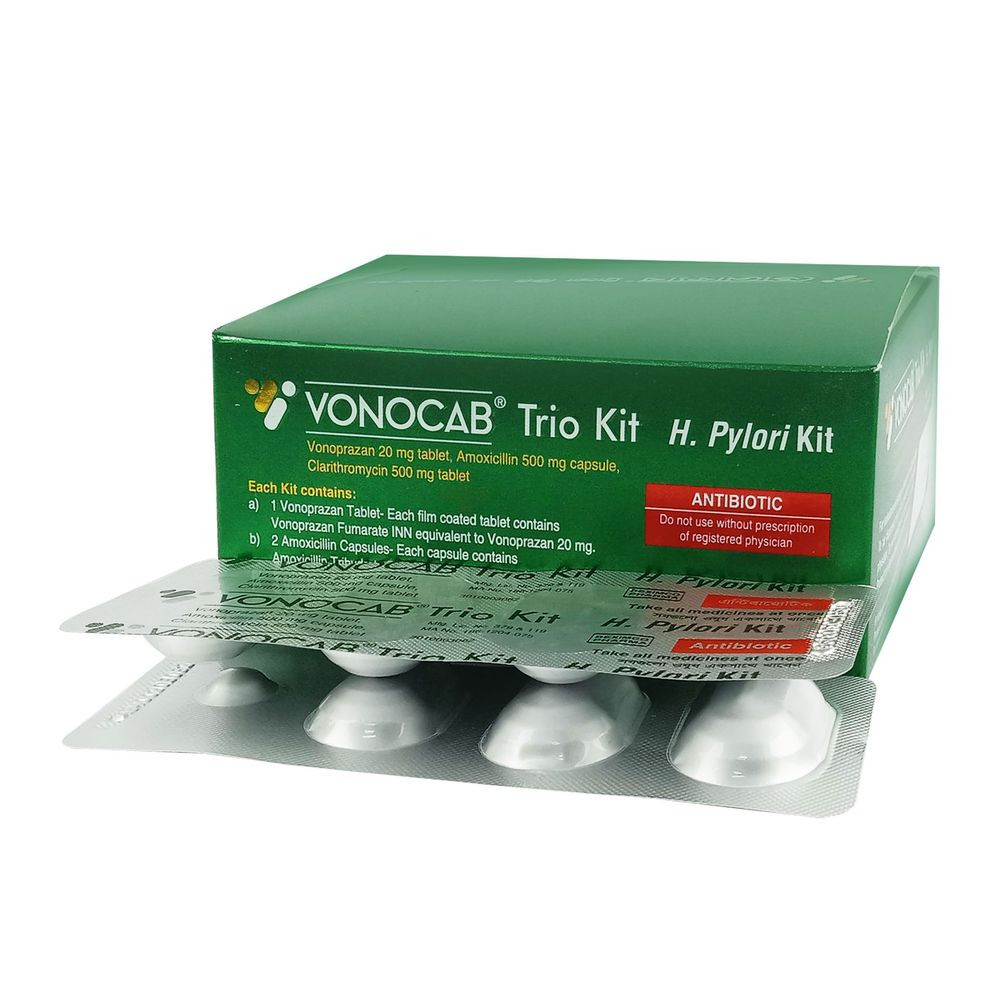
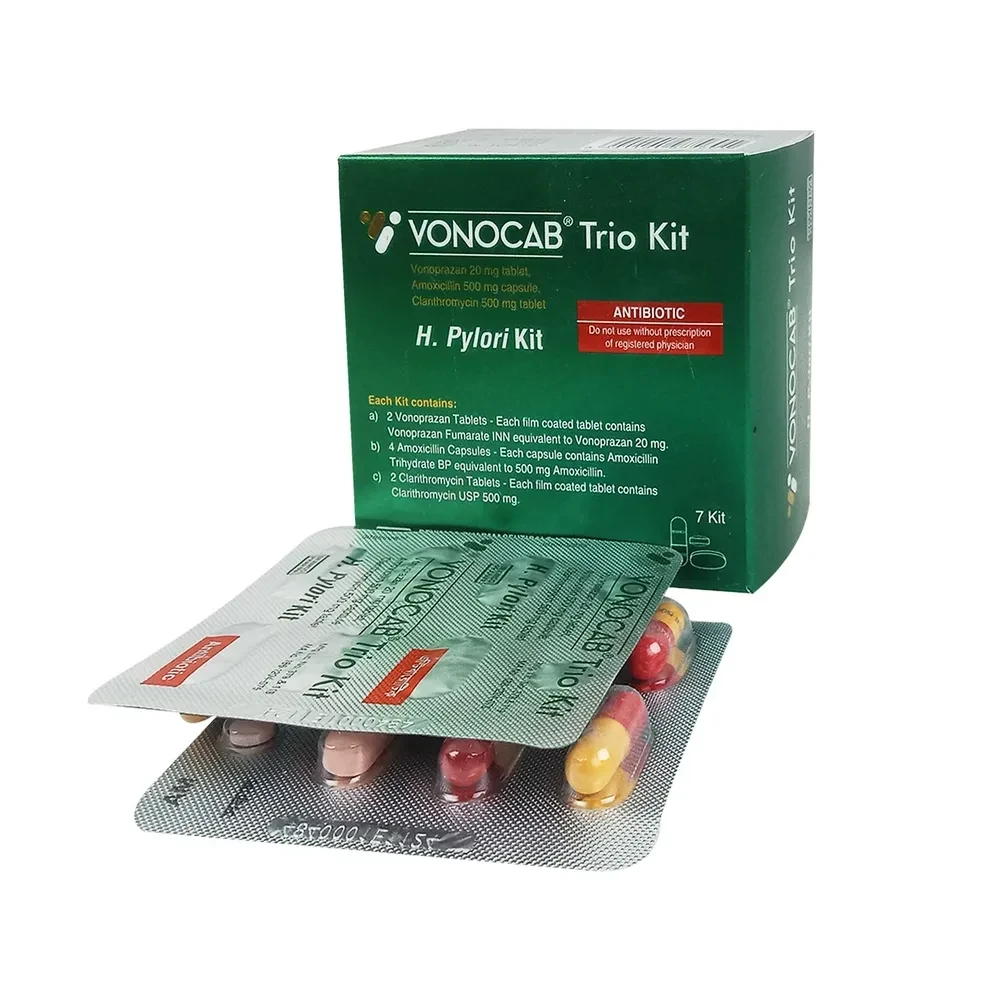
Vonocab Trio Tablet
Pack Images
20 mg+1000 mg+500 mg
4 tablets kit:
৳ 60.00
(14's pack: ৳ 840.00)
Indications
Vonocab Trio, is a standard triple therapy regimen, is indicated in the eradication of H. pylori in active chronic gastritis, duodenal and gastric ulcers.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Composition
Each Vonokit, Vonocab Trio, Helicon V kit blister strip contains-
- One Vonoprazan 20 mg tablet (as Vonoprazan Fumarate INN)
- Two Amoxicillin 500 mg capsules (as Amoxicillin Trihydrate BP)
- One Clarithromycin 500 mg tablet (as Clarithromycin USP)
- Two Vonoprazan 20 mg Tablets (Each film coated tablet contains Vonoprazan Fumarate INN equivalent to Vonoprazan 20 mg)
- Four Amoxicillin 500 mg Capsules (Each capsule contains Amoxicillin Trihydrate BP equivalent to Amoxicillin 500 mg)
- Two Clarithromycin 500 mg Tablets (Each film coated tablet contains Clarithromycin USP 500 mg
Pharmacology
Helicobacter pylori (H. pylori) is implicated in the etiology of gastritis & peptic ulcer. The standard triple therapy regimen contains 20 mg vonoprazan tablet, 500 mg amoxicillin capsule and 500 mg clarithromycin tablet for oral administration is effective for eradicating H. pylori.
Vonoprazan suppresses basal and stimulated gastric acid secretion at the secretory surface of the gastric parietal cell through inhibition of the H+ K+ -ATPase enzyme system in a potassium competitive manner. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, vonoprazan has been characterized as a type of gastric proton-pump inhibitor, in that it blocks the final step of acid production. Vonoprazan does not require activation by acid. Vonoprazan may selectively concentrate in the parietal cells in both the resting and stimulated states. Vonoprazan binds to the active proton pumps in a noncovalent and reversible manner. Amoxicillin is an antibacterial drug. Clarithromycin is a macrolide antimicrobial drug. Acid suppression enhances the replication of H. pylori bacteria and the stability and effectiveness of antimicrobials in the treatment of H. pylori infection.
Vonoprazan suppresses basal and stimulated gastric acid secretion at the secretory surface of the gastric parietal cell through inhibition of the H+ K+ -ATPase enzyme system in a potassium competitive manner. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, vonoprazan has been characterized as a type of gastric proton-pump inhibitor, in that it blocks the final step of acid production. Vonoprazan does not require activation by acid. Vonoprazan may selectively concentrate in the parietal cells in both the resting and stimulated states. Vonoprazan binds to the active proton pumps in a noncovalent and reversible manner. Amoxicillin is an antibacterial drug. Clarithromycin is a macrolide antimicrobial drug. Acid suppression enhances the replication of H. pylori bacteria and the stability and effectiveness of antimicrobials in the treatment of H. pylori infection.
Dosage & Administration
The recommended dosage regimen is-
Geriatrics: The standard triple therapy regimen increased risk of torsade de pointes due to the clarithromycin component.
Renal Impairment: Avoid use in severe renal impairment.
Hepatic Impairment: Avoid use in moderate and severe hepatic impairment.
- Vonoprazan 20 mg
- Amoxicillin 1000 mg
- Clarithromycin 500 mg
Geriatrics: The standard triple therapy regimen increased risk of torsade de pointes due to the clarithromycin component.
Renal Impairment: Avoid use in severe renal impairment.
Hepatic Impairment: Avoid use in moderate and severe hepatic impairment.
* রেজিস্টার্ড চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন
Interaction
Triple therapy regimen should be used with caution when co-administrated with following drugs: Tacrolimus, cyclosporine, clopidogrel, citalopram, cilostazol, probenecid, allopurinol, itraconazole, ketoconazole, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, saquinavir, ritonavir, etravirine, rilpivirine-containing products, atazanavir, nelfinavir.
Contraindications
The standard triple therapy regimen is contraindicated in patients with known hypersensitivity to vonoprazan, amoxicillin or any other betalactams, clarithromycin or any other macrolide antimicrobials
Side Effects
Most common adverse reactions (>2%) were dysgeusia, diarrhea, vulvovaginal candidiasis, headache, abdominal pain, hypertension and nasopharyngitis.
Pregnancy & Lactation
Based on findings from animal studies and observational studies in pregnant women with use of clarithromycin, use of this triple pack is not recommended in pregnant women except in clinical circumstances where no alternative therapy is appropriate. There are no data regarding the presence of vonoprazan in human milk, the effects on the breastfed infant or the effects on milk production. Vonoprazan and its metabolites are present in rat milk.
Precautions & Warnings
Hypersensitivity Reactions: Serious and occasionally fatal reactions (e.g., anaphylaxis) have been reported with components of this triple pack. If hypersensitivity reactions occur, discontinue and institute immediate therapy (e.g., anaphylaxis management).
Severe Cutaneous Adverse Reactions (SCAR): Discontinue this pack at the first signs or symptoms of SCAR or other signs of hypersensitivity and consider further evaluation.
Clostridioides difficile-associated diarrhea (CDAD): Evaluate if diarrhea occurs with this pack and
Due to the Clarithromycin Component:
QT Prolongation: Avoid this pack in patients with known QT prolongation or receiving drugs known to prolong the QT interval, ventricular arrhythmia (torsades de pointes), hypokalemia/hypomagnesemia, significant bradycardia, or taking Class IA or III antiarrhythmics.
Hepatotoxicity: Discontinue if signs and symptoms of hepatitis occur with this pack.
Serious adverse reactions due to concomitant use with other drugs: Serious adverse reactions can occur with this pack due to drug interactions of clarithromycin with colchicine, some lipid lowering agents, some calcium channel blockers, and other drugs.
Embryo-Fetal Toxicity: Based on the findings from animal studies and human observational studies in pregnant women treated with clarithromycin, this pack is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate.
Myasthenia Gravis: Exacerbation of myasthenia gravis can occur with this pack since it has been reported in patients receiving clarithromycin tablets.
Severe Cutaneous Adverse Reactions (SCAR): Discontinue this pack at the first signs or symptoms of SCAR or other signs of hypersensitivity and consider further evaluation.
Clostridioides difficile-associated diarrhea (CDAD): Evaluate if diarrhea occurs with this pack and
Due to the Clarithromycin Component:
QT Prolongation: Avoid this pack in patients with known QT prolongation or receiving drugs known to prolong the QT interval, ventricular arrhythmia (torsades de pointes), hypokalemia/hypomagnesemia, significant bradycardia, or taking Class IA or III antiarrhythmics.
Hepatotoxicity: Discontinue if signs and symptoms of hepatitis occur with this pack.
Serious adverse reactions due to concomitant use with other drugs: Serious adverse reactions can occur with this pack due to drug interactions of clarithromycin with colchicine, some lipid lowering agents, some calcium channel blockers, and other drugs.
Embryo-Fetal Toxicity: Based on the findings from animal studies and human observational studies in pregnant women treated with clarithromycin, this pack is not recommended for use in pregnant women except in clinical circumstances where no alternative therapy is appropriate.
Myasthenia Gravis: Exacerbation of myasthenia gravis can occur with this pack since it has been reported in patients receiving clarithromycin tablets.
Overdose Effects
If overdose occurs, should consult with doctor immediately.
Therapeutic Class
Anti H. pylori drugs
Storage Conditions
Do not store above 30°C. Keep in a dry place. Protect from light and keep out of the reach of children.
Pack Images: Vonocab Trio 20 mg Tablet